If we can get cancer cells to die faster than they grow, we can shrink cancer tumours, and even – eventually – fully cure them. And a shrinking or vanishing tumour can't kill us. This is plain logic and the aim of our Mosaic Method.
It's of course far harder to do than say. How do we increase the death rate of cancer cells? How do we decrease their growth rate? The Mosaic Method uses multiple attack points to try and achieve both at the same time.
Today, we'll zoom in on a part of step six of the method – namely, the elimination of oxalate from the diet. In the Mosaic Method Guide (which you can read for free at https://www.marchward.com/mosaic), we've only barely touched on why oxalates are so counterproductive, when trying to outlive cancer.
So, I want to expand on that.
Oxalate is a compound commonly found in plant foods. It's also made by our own bodies – primarily by the liver. We can influence both sources of oxalate, but why would we want to? What makes oxalate harmful to cancer patients?
For cancer patients oxalate is mainly of interest in its role as a substance causing inflammation and mitochondrial dysfunction (1–5). Chronic inflammation and mitochondrial dysfunction stand on two spots of a vicious cycle promoting one another (6–7). Mitochondrial dysfunction is the primary cause in the development of cancer and restoring mitochondrial metabolism through various means has been shown to reduce cancer growth and thus patient mortality (8–13).
Though I've written on this at length in the prior post 'How Our Dad Is Outliving Cancer', it bears repeating, what mitochondria are and how they influence cells and consequently how their disruption can cause cancer.
Mitochondria (Fig. 1 – 3) are small compartments within cells, which primarily handle energy metabolism and various quality control measures (15–17). There exists an alternative energy metabolism in the cytosol (Fig. 1 – 2) – namely sugar fermentation (technically known as 'glycolysis') –, but this alternative energy metabolism is highly inefficient, both in terms of energy produced and waste generated. The most potent of the quality control measures – the so-termed apoptosis, a cell suicide – can only be initiated in two ways: The mitochondria can determine the cell they reside in to be too damaged and then initiate apoptosis, or specialised cells of the immune system can detect infected or defective cells and kill them.
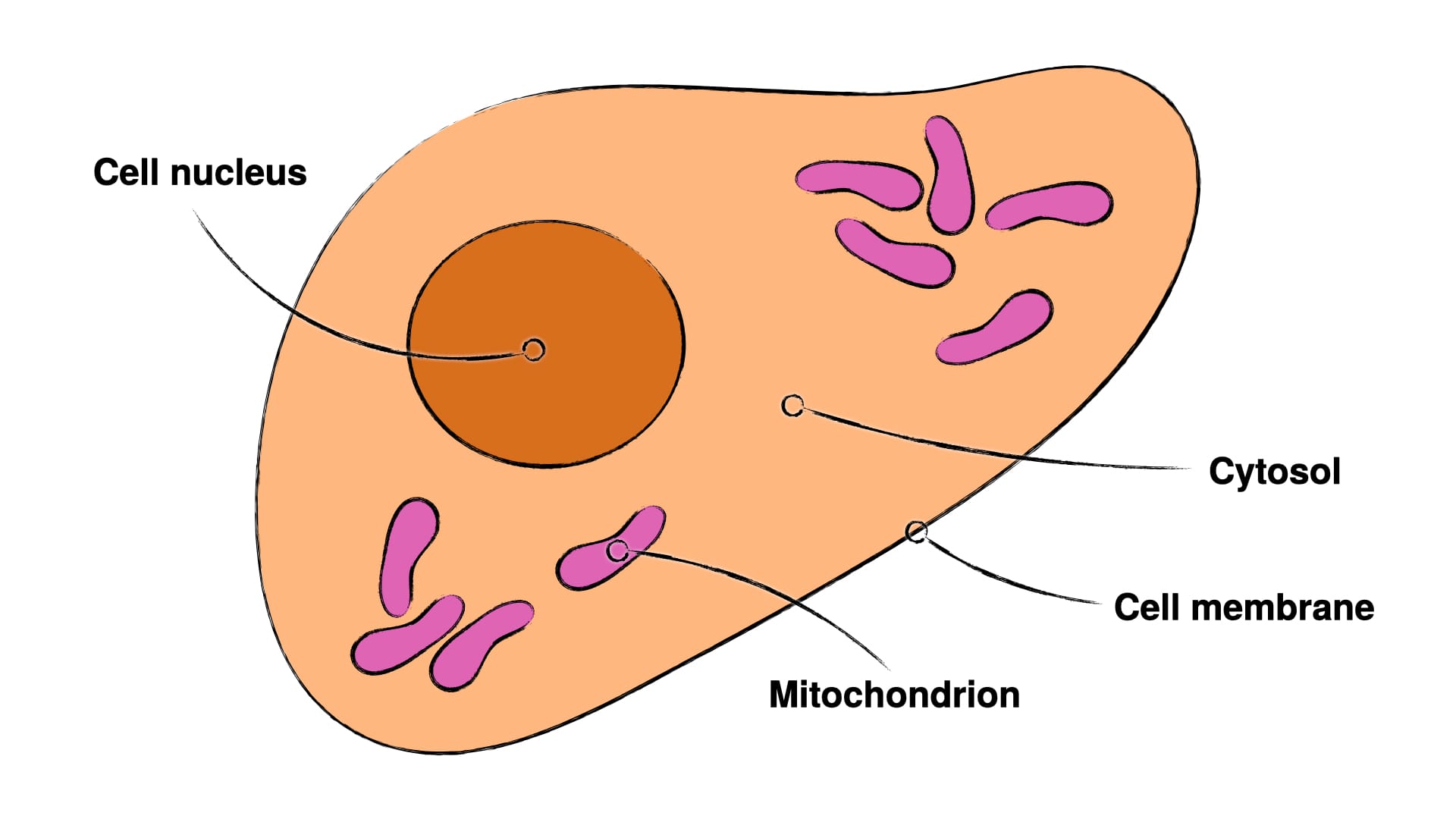
As this post is on oxalates and they seem to not have a whole lot to do with immune evasion, we'll focus on the mitochondria. Since mitochondria are the primary cause of cancer and since they can kill defective cells, logic dictates, that if we can restore mitochondrial function, the restored mitochondria will either enable the cancer cell to heal or kill it outright, if it's too damaged. And indeed this logic is reflected in the research (5, 8–10).
We publish new articles, whenever we've synthesised new findings to a usable degree. You can subscribe to receive articles for free.
As I've already mentioned, oxalates cause both inflammation and disrupt mitochondrial function. It would thus stand to reason, that oxalates can directly cause cancer, when found in appropriate concentrations. And wouldn't you know it, this is absolutely the case.
It's long since been established, that some types of breast cancer – specifically the highly aggressive HER2-positive breast cancers – are correlated with deposits of insoluble oxalates (18). Now, correlation isn't enough as evidence, but it was enough of an indication to leave further research wished for.
And so, Castellaro et al. studied oxalates in the context of breast tissue cells and breast cancer cells. Causation was studied more directly by exposing mice to oxalates and checking for breast cancer development.
They found, that 3 weeks of exposure to oxalate of healthy breast tissue cells led to about an 8-times greater growth rate over non-exposed cells. What's worse, is that injection of calcium oxalate (an insoluble oxalate) into the breast led to cancer development also in mice and to a 100% mortality rate within 90 days. Injection of the same amount of oxalate in the form of the soluble potassium oxalate lead to far quicker 100% mortality within 30 days. Interestingly, however, injection of the same soluble oxalate into the back of the mice led to no fatalities within the studied time-frame.
To me this seems to indicate, that oxalates made by our own body are hardly an issue, as they are primarily produced by liver and oxalates seem to be cancer-promoting in a tissue-specific way. There's more research needed on the effect upon other cells, although it is known, that oxalates trigger and promote cancer-like behaviour in other tissues, such as kidneys and lungs. This seems to indicate a broader potential in causing cancer development – and thus also stopping the healing of cancer (18–19).
Mind, that the research on the interactions of oxalates and cancer has been most heavily focused on study in breast cancer. Nonetheless, it's known, that oxalate has negative consequences for certain immune cells and inflammatory signalling (3–4). This has also been shown to follow dietary consumption of oxalates specifically, thus bringing me back to the importance of abstaining from dietary oxalates (20).
It seems, that the liver has some way of coping with oxalate, and indeed the liver is incredibly apt at handling toxins in general – given the fact, that handling toxins is one of its primary roles, this is hardly surprising. Still, the liver can have too much oxalate and inhibition of oxalate production then improves liver health (21).
Since modulating oxalate production pharmacologically is beyond my pay grade, we'll focus on dietary oxalate – as it's absorbed into the blood in the small intestine and then distributed throughout the body without much control over tissue-specific distribution – and on dietary factors, which promote oxalate production.
So let's look at that.
We don't take sponsoring or display advertisements in order to keep us free from conflicts of interest. If you've found this valuable, you can help us by supporting our fight against cancer financially.
Where do we find oxalates in food stuffs? We find oxalates almost exclusively in plant foods. Now, it would be simplest to just abstain entirely from plant foods. But, but, I know. It's simple, but not necessarily easy. So, here's something interesting: processing of oxalate-rich food by way of steeping and boiling and consumption of oxalate-rich food together with calcium-rich food significantly lowers oxalate content in and absorption from the food (23–24).
So, if I were you, I'd abstain as best as possible from plant food stuffs, but if you then do eat them, eat them with dairy or egg shells and only after long (48+ hours) steeping in water. If you like coffee or tea, for example, I'd highly recommend to use some milk in both or perhaps sprinkle some powdered egg shells in there. The latter won't really affect the taste, but if you find it unconscionable to treat your tea or coffee to powdered egg shells, consider just taking a tipfull of a teaspoon with water before hand.
Now, what foods promote oxalate production and what foods inhibit it? Well, the evidence for this is indirect. Studies looking at the influence of diet on oxalate metabolism tend to focus on urinary oxalate load, not body-internal oxalate load, as they mostly concern themselves with kidney stone disease, for which urinary oxalate is causally implied (25–27). Urinary oxalate secretion does however seem to be influenced by gelatine, fructose (fruit sugar), and vitamin B6 deficiency. As vitamin B6 is found in large enough quantities and high bioavailability in meat and even some plant foods, I don't think vitamin B6 is an issue (28). You may find a personal deficiency upon blood imaging.
All in all, I think it's wisest to stay away from dietary oxalates as best as is possible. I don't necessarily think other measures are necessary with regard to body-internal oxalates and their potential influence on cancer.
Health and healing to you,
Merlin.
P.S.: You can get the full Mosaic Method Guide for free under https://www.marchward.com/mosaic.
References
- Cao, L.C., Honeyman, T.W., Cooney, R., Kennington, L., Scheid, C.R., and Jonassen, J.A. (2004). Mitochondrial dysfunction is a primary event in renal cell oxalate toxicity. Kidney Int 66, 1890-1900. 10.1111/j.1523-1755.2004.00963.x.
- Chaiyarit, S., and Thongboonkerd, V. (2020). Mitochondrial Dysfunction and Kidney Stone Disease. Frontiers in Physiology 11. 10.3389/fphys.2020.566506.
- Kumar, P., Laurence, E., Crossman, D.K., Assimos, D.G., Murphy, M.P., and Mitchell, T. (2023). Oxalate disrupts monocyte and macrophage cellular function via Interleukin-10 and mitochondrial reactive oxygen species (ROS) signaling. Redox Biology 67, 102919. 10.1016/j.redox.2023.102919.
- Patel, M., Yarlagadda, V., Adedoyin, O., Saini, V., Assimos, D.G., Holmes, R.P., and Mitchell, T. (2018). Oxalate induces mitochondrial dysfunction and disrupts redox homeostasis in a human monocyte derived cell line. Redox Biol 15, 207-215. 10.1016/j.redox.2017.12.003.
- Zhang, J., Wang, Q., Xu, C., Lu, Y., Hu, H., Qin, B., Wang, Y., He, D., Li, C., Yu, X., et al. (2017). MitoTEMPO Prevents Oxalate Induced Injury in NRK-52E Cells via Inhibiting Mitochondrial Dysfunction and Modulating Oxidative Stress. Oxid Med Cell Longev 2017, 7528090. 10.1155/2017/7528090.
- López-Armada, M.J., Riveiro-Naveira, R.R., Vaamonde-García, C., and Valcárcel-Ares, M.N. (2013). Mitochondrial dysfunction and the inflammatory response. Mitochondrion 13, 106-118. 10.1016/j.mito.2013.01.003.
- Orekhov, A.N., Summerhill, V.I., Khotina, V.A., Popov, M.A., Uzokov, J.K., and Sukhorukov, V.N. (2023). Role of Mitochondria in the Chronification of Inflammation: Focus on Dysfunctional Mitophagy and Mitochondrial DNA Mutations. Gene Expression 22, 329-344. 10.14218/ge.2023.00061.
- Bonnet, S., Archer, S.L., Allalunis-Turner, J., Haromy, A., Beaulieu, C., Thompson, R., Lee, C.T., Lopaschuk, G.D., Puttagunta, L., Bonnet, S., et al. (2007). A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell 11, 37-51. 10.1016/j.ccr.2006.10.020.
- Seyfried, T.N., and Chinopoulos, C. (2021). Can the Mitochondrial Metabolic Theory Explain Better the Origin and Management of Cancer than Can the Somatic Mutation Theory? Metabolites 11.
- Michelakis, E.D., Webster, L., and Mackey, J.R. (2008). Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br J Cancer 99, 989-994. 10.1038/sj.bjc.6604554.
- Seyfried, T.N., Arismendi-Morillo, G.J., Mukherjee, P., and Chinopoulos, C. (2020). On the Origin of ATP Synthesis in Cancer. iScience 23.
- Seyfried, T.N., and Huysentruyt, L.C. (2013). On the origin of cancer metastasis. Critical reviews in oncogenesis 18 1-2, 43-73.
- Seyfried, T.N., and Shelton, L.M. (2010). Cancer as a metabolic disease. Nutrition & Metabolism 7, 7 - 7.
- Deretic, V., and Kroemer, G. (2022). Autophagy in metabolism and quality control: opposing, complementary or interlinked functions? Autophagy 18, 283-292. 10.1080/15548627.2021.1933742.
- Alberts, B., Johnson, A., Lewis, J., Morgan, D., Raff, M., Roberts, K., and Walter, P. (2015). Cell Chemistry and Bioenergetics. In Molecular Biology of the Cell, (Garland Science, Taylor & Francis Group, LLC), pp. 43-108.
- Alberts, B., Johnson, A., Lewis, J., Morgan, D., Raff, M., Roberts, K., and Walter, P. (2015). Energy Conversion: Mitochondria and Chloroplasts. In Molecular Biology of the Cell, (Garland Science, Taylor & Francis Group, LLC), pp. 753-812.
- Alberts, B., Johnson, A., Lewis, J., Morgan, D., Raff, M., Roberts, K., and Walter, P. (2015). Cell Death. In Molecular Biology of the Cell, (Garland Science, Taylor & Francis Group, LLC), pp. 1021-1034.
- Peerapen, P., Boonmark, W., Putpeerawit, P., and Thongboonkerd, V. (2022). Calcium oxalate crystals trigger epithelial-mesenchymal transition and carcinogenic features in renal cells: a crossroad between kidney stone disease and renal cancer. Exp Hematol Oncol 11, 62. 10.1186/s40164-022-00320-y.
- Zeng, Z., Xu, S., Wang, F., Peng, X., Zhang, W., Zhan, Y., Ding, Y., Liu, Z., and Liang, L. (2022). HAO1-mediated oxalate metabolism promotes lung pre-metastatic niche formation by inducing neutrophil extracellular traps. Oncogene 41, 3719-3731. 10.1038/s41388-022-02248-3.
- Castellaro, A.M., Tonda, A., Cejas, H.H., Ferreyra, H., Caputto, B.L., Pucci, O.A., and Gil, G.A. (2015). Oxalate induces breast cancer. BMC Cancer 15, 761. 10.1186/s12885-015-1747-2.
- Kumar, P., Patel, M., Oster, R.A., Yarlagadda, V., Ambrosetti, A., Assimos, D.G., and Mitchell, T. (2021). Dietary Oxalate Loading Impacts Monocyte Metabolism and Inflammatory Signaling in Humans. Front Immunol 12, 617508. 10.3389/fimmu.2021.617508.
- Das, S., Finney, A.C., Anand, S.K., Rohilla, S., Liu, Y., Pandey, N., Ghrayeb, A., Kumar, D., Nunez, K., Liu, Z., et al. (2024). Inhibition of hepatic oxalate overproduction ameliorates metabolic dysfunction-associated steatohepatitis. Nature Metabolism 6, 1939-1962. 10.1038/s42255-024-01134-4.
- Abera, S., Yohannes, W., and Chandravanshi, B.S. (2023). Effect of Processing Methods on Antinutritional Factors (Oxalate, Phytate, and Tannin) and Their Interaction with Minerals (Calcium, Iron, and Zinc) in Red, White, and Black Kidney Beans. International Journal of Analytical Chemistry 2023, 6762027. 10.1155/2023/6762027.
- Huynh, N.K., Nguyen, D.H.M., and Nguyen, H.V.H. (2022). Effects of processing on oxalate contents in plant foods: A review. Journal of Food Composition and Analysis 112, 104685. 10.1016/j.jfca.2022.104685.
- Knight, J., Jiang, J., Assimos, D.G., and Holmes, R.P. (2006). Hydroxyproline ingestion and urinary oxalate and glycolate excretion. Kidney international 70 11, 1929-1934.
- Mitwalli, A., Ayiomamitis, A., Grass, L., and Oreopoulos, D.G. (1988). Control of hyperoxaluria with large doses of pyridoxine in patients with kidney stones. International Urology and Nephrology 20, 353-359.
- Taylor, E.N., Curhan, G.C., and Curhan, G.C. (2008). Fructose consumption and the risk of kidney stones. Kidney international 73 2, 207-212.
- Reynolds, R.D. (1988). Bioavailability of vitamin B-6 from plant foods. The American Journal of Clinical Nutrition 48, 863-867. 10.1093/ajcn/48.3.863.