Correction!!!


Pathology from persistent spike protein causes both "long-COVID" and anti-SARS-CoV-2 vaccine injuries.1 It's difficult to understand how packaging this pathogenic protein in a vaccine was ever approved, unless we understand, that the entire approval flow for the lipid nanoparticle–encapsulated mRNA gene therapeutic anti-SARS-CoV-2 vaccines was shoddy.
Many of the claims, upon which approval was based, have since been called into question in independent experiments. Furthermore, approval was made without available long-term data, both for the specific drugs in question, but also for the technology at large.
That this was a horrendous failure on part of regulators, goes without saying, and that those involved in the obfuscation of the adverse effects of the vaccines remain at large, shows us the sordid state of our societies.2 As of September 2022, 68% of the global population has received at least one dose of anti-SARS-CoV-2 vaccination, roughly 30% of which were administered the novel vaccines using lipid nanoparticle–encapsulated synthetic mRNA.
Two primary claims were made about these novel vaccines in support of their safety:
- That the injection would stay at the injection site.
- That they were inherently labile and would degrade within minutes.
Both these claims have been discredited since by virtue of spike-coding mRNA and the spike protein itself being found in organs and tissues far off from the injection site with an average persistence of 105 days in patients suffering from post-vaccination pathologies.1 That is the average. The upper bound for persistence is unclear and probably depends on multiple physiological factors.
As teased above, spike protein itself is pathogenic. This is probably part of the reason why the COVID-19 vaccine has much higher occurrence of adverse effects compared to other vaccines, including having been involved in the killing of patients.1–3
So, in this article, we'll look at exactly how spike protein harms patients, its significance to cancer and other chronic ailments, and then what therapeutic approaches we have available to support its clearance from the body to reinstate full patient health.
This article will mostly be interesting to healthcare practitioners and patients suffering acutely from long-COVID, post-vaccination injury, or latent infections with SARS-CoV-2.
We publish new articles, whenever we've synthesised new findings to a usable degree. You can subscribe to receive articles for free.
How Spike Protein Harms Patients
This section will be a bit technical, but we'll go over the most important things from this section, when discussing the significance of spike-protein pathology for cancer and other chronic ailments.
Spike protein causes harm in multiple organ systems and in multiple ways.3 Spike protein lowers blood-brain barrier function, which can lead to central nervous system inflammation and following neuropsychiatric symptoms. It interacts with receptors used in immune signalling in a way, that promotes immune senescence under prolonged activation. It interacts with receptors used in controlling blood clotting, which can lead to thrombosis, haemolytic anaemia, myocarditis, and pericarditis. It interacts with an oestrogen receptor, which can cause menstrual abnormalities. It can lead to mitochondrial damage and thus metabolic dysregulation. It down-regulates the function of certain neural transmitter receptors, which makes motor and autonomous nervous signalling more difficult. And lastly, it's indicated to inhibit certain tumour suppressor genes, who's task it is to keep cell integrity under surveillance and terminate the cell, should cell integrity fall below a critical threshold.
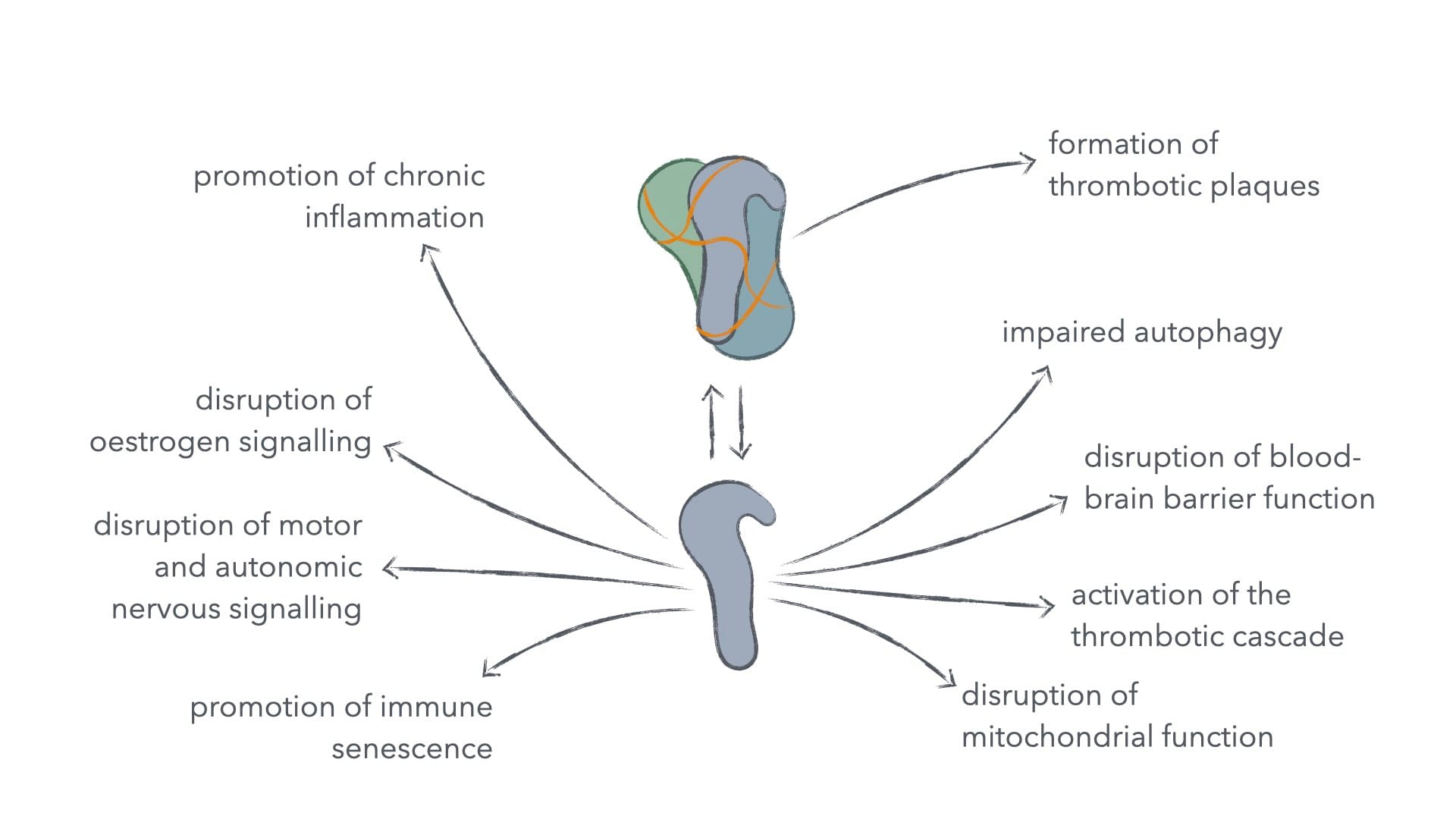
Figure 1: How spike protein interacts with various bodily systems.
Inhibition of tumour suppressor genes, engendering immune senescence, and metabolic dysregulation are probably the most significant to cancer development and treatment, but also for other ailments it becomes essential to support clearance of spike protein, as to free the body from long-term negative effects from SARS-CoV-2 infection and the catastrophically subpar mRNA-based anti-SARS-CoV-2 vaccines.
Its Significance in Cancer and Other Chronic Ailments
This section will be subdivided by mechanism of spike-protein pathology.
Changes to Blood-Clotting and Formation of Protein Aggregates
Changes in blood clotting can lead to thrombosis, which can block small blood vessels and capillaries. If such blood clots aren't efficiently removed, they lead to hypoxia (oxygen starvation). During low-grade hypoxia, mitochondrial function is boosted to improve aerobic metabolism despite dearth of oxygen, but if hypoxia continues to worsen mitochondrial function becomes largely suspended, and the cell either switches to fermentation for energy generation or dies.4
The waste product of our fermentation is acidic and in environments, where waste product clearance is compromised – such as in our thrombosis-compromised tissue –, this acidity builds up, leading to enhanced genomic instability of affected cells and the inactivation of incident immune cells, trying to clean up debris from dead cells.5–9 This in turn gives us a highly cancer-promoting environment.
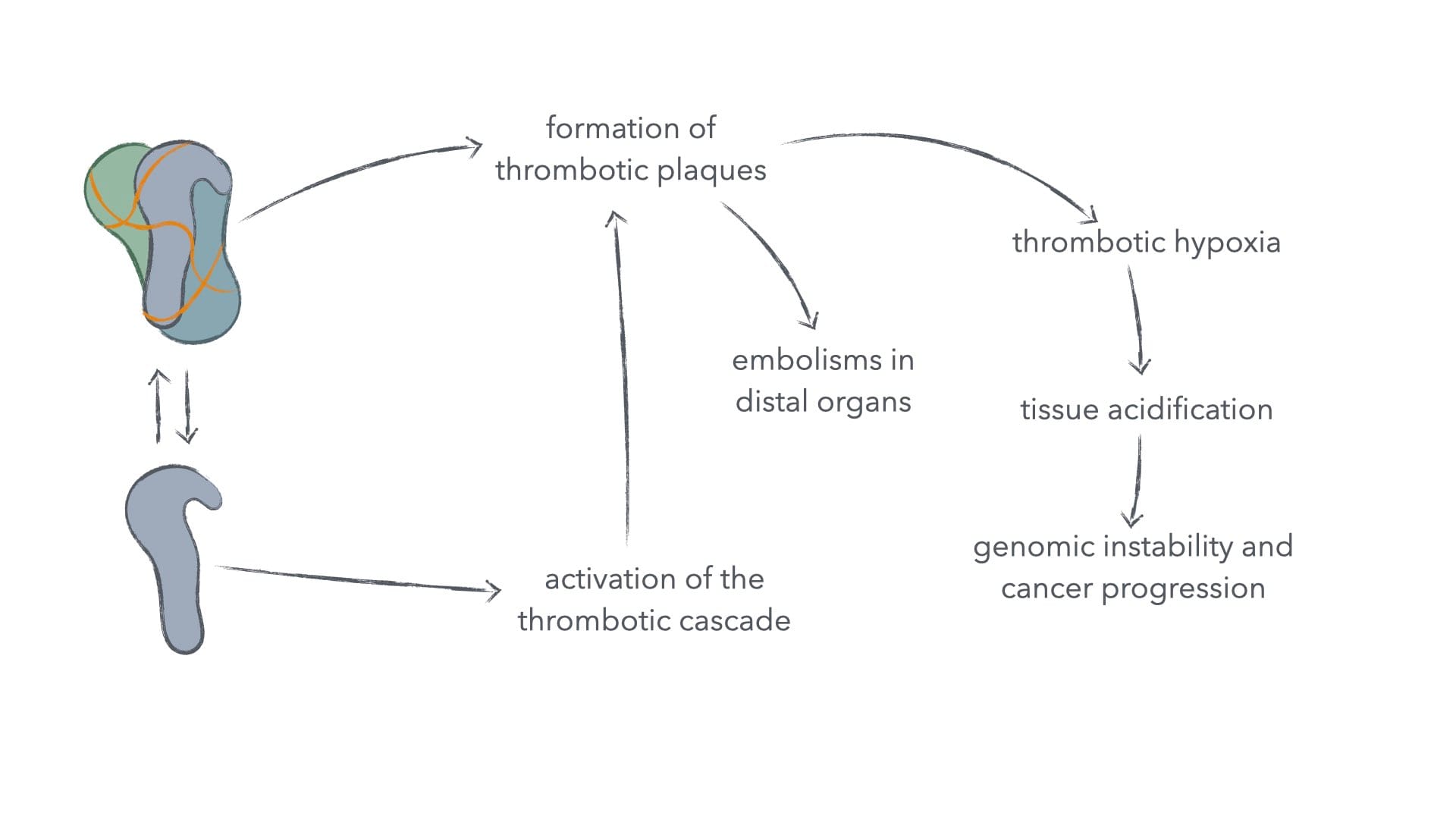
Figure 2: Spike protein in clotting and its consequences for cancer and other ailments.
Such thrombotic plaques can of course also directly cause embolism in all organs, should they tear free from their site of creation and travel in the blood.10 Pulmonary embolism seems to be the most clinically relevant in patient mortality from spike protein, whether delivered by infection or vaccination, as spike protein alone suffices in the initiation of thrombosis.10–11 Spike protein has also been found to indirectly (via fibrin) drive neuroinflammation, innate immune activation in the brain, and death of neurons independently of infection.12
We don't take sponsoring or display advertisements in order to keep us free from conflicts of interest. If you've found this valuable, you can help us by supporting our fight against cancer financially. Without your support we'll eventually have to terminate our efforts.
Spike Protein–Induced Mitochondrial Damage
Spike protein also induces mitochondrial damage via a pathway independent from the above thrombotic-hypoxic pathway.1 The mechanism by which spike protein does so has multiple prongs.
Spike protein directly damages mitochondria, lowering their metabolic rate and increasing the generation of reactive oxygen species – a highly reactive and toxic metabolic byproduct.1 It also inhibits the pathway, by which such damaged mitochondria are normally destroyed and their materials recycled to form new mitochondria – a process known as mitophagy. Interestingly, this characteristic of spike protein of inhibiting mitophagy is at least partially counteracted by another protein encoded in the whole SARS-CoV-2 genome, which actually stimulates mitophagy.
Thus, whilst the problem of inducing mitochondrial damage isn't exclusive to the vaccines, the problem of sustained mitochondrial and thus metabolic dysfunction is especially bad in the mRNA vaccines and less prevalent in whole-virus infections.
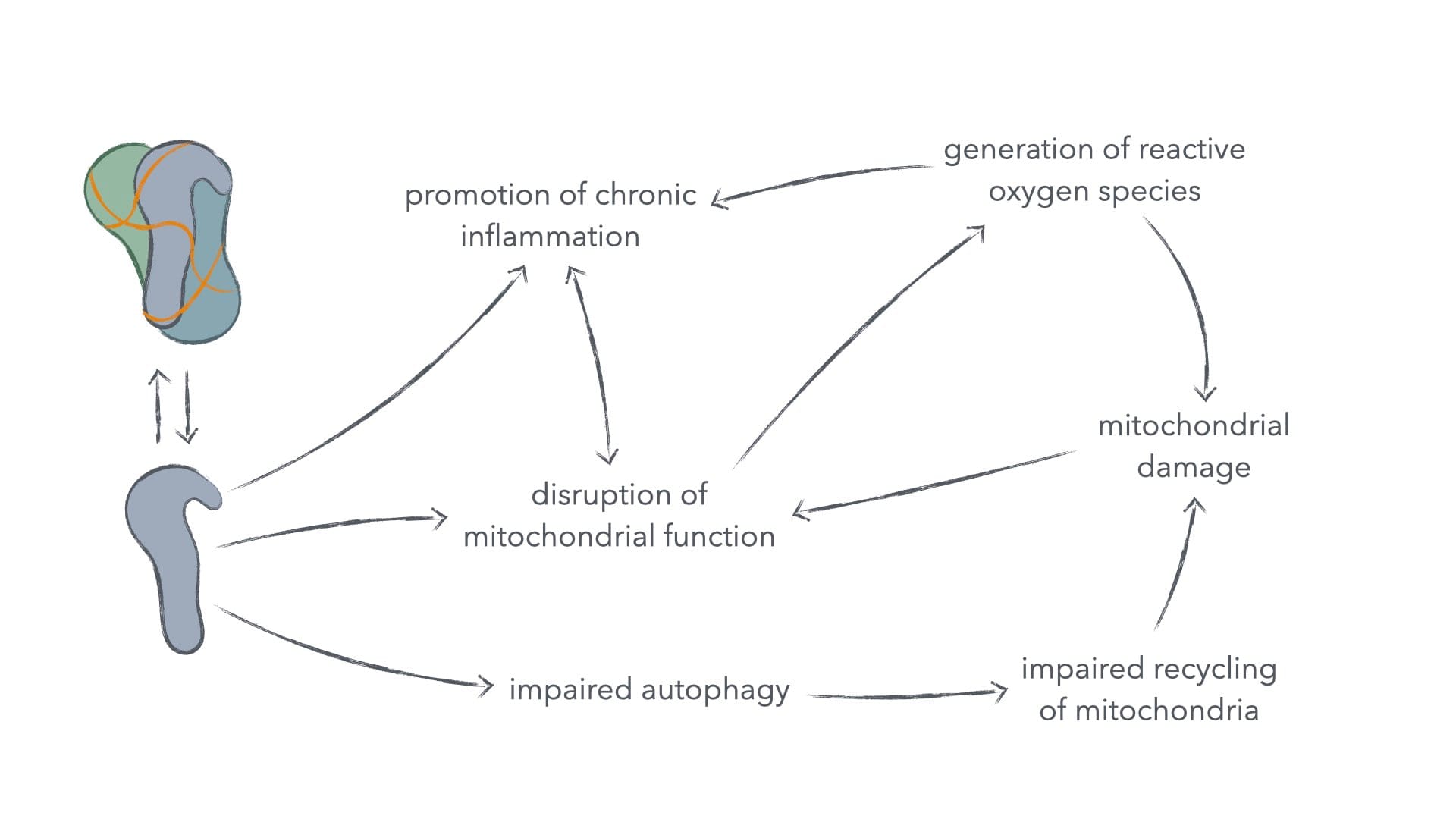
Figure 3: Spike protein–induced mitochondrial damage and its consequences for cancer and other ailments.
Chronic mitochondrial dysfunction is heavily involved in the development and progression of cancer.13–14 It's also implicated in insulin resistance and chronic inflammation.15–17 Insulin resistance itself is a risk factor in type-2 diabetes, hypertension, cardiovascular disease, neuropathy, nephropathy, and retinopathy. The negative effects of chronic inflammation, we'll discuss in the next subsection, as spike protein also directly drives chronic-inflammatory immune senescence as well as other chronic-inflammatory complications.
Chronic-Inflammatory Immune senescence
Spike protein specifically interacts with the toll-like receptors 2 and 4 (TLR-2, -4). These receptors have important and versatile functions within the immune system.18 One of the downstream effects of TLR induction on dendritic cells (a type of immune response–directing cell) is the expression of interleukin 1, interleukin 6 (IL-6), and tumour necrosis factor alpha (TNF-a). Whilst this signalling profile is initially helpful for mounting an immune response, the chronic expression of IL-6 is immunosuppressive.
In fact, chronic IL-6 expression is used by mesotheliomata and many other cancers to induce immune senescence and thus enables them to evade immune destruction.19–20 What's more, the precise expression profile of IL-6 and TNF-a is exhibited by senescent immune cells in the microenvironment of immune evasive lung cancers.21
This is disastrous for our mounting an effective anti-cancer immune response, but this also has implications in other chronic-inflammatory diseases. For example, it's logical that this immunosuppressive signalling enables persistent infection of the tonsil.22 Such persistent infection can lead to chronic tonsillar complications, so much so, that in one study 1 in 4 patients put through tonsillectomy have such persistent spike-protein to thank for their complications. It seems likely, that prolonged exposure to spike protein from vaccinations can indeed have the opposite effect of what we would expect from a vaccine. Catastrophically, this is precisely what we see in patients. Spike protein from SARS-CoV-2 vaccinations are actually suppressive to innate immune system function.23
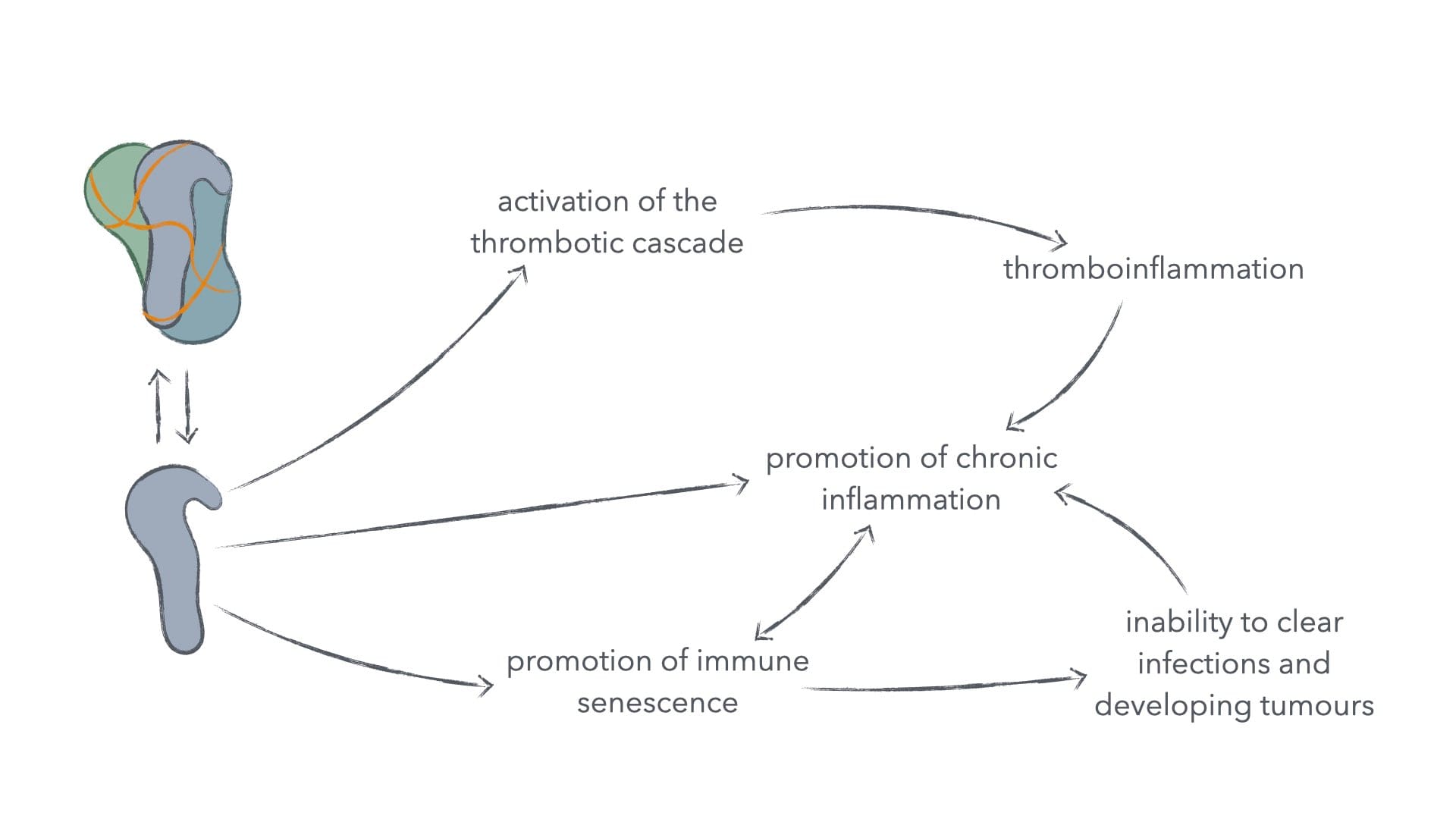
Figure 4: Immunosuppressive activity of spike protein and its consequences.
Such suppressed innate immune function isn't just bad for mounting an effective anticancer immune response, but for mounting any effective immune response, for the innate immune system is required to properly activate and co-stimulate the adaptive immune system, which in turn offers much more specialised immune cells to neutralise free viruses and destroy both infected and defective cells.24 This would in turn be beneficial to cancer and pathogens, and thus detrimental to the patients.
Naturally, the question arises about what we can do against this.
Therapeutic Approaches to Counteract Persistent Spike Protein
There are multiple things we can do to stop the negative effects of persistent spike protein. The most obvious is that we can clear the spike protein from our body. Now, this itself can be done directly – by the administration of certain enzymes, which de-aggregate and digest spike protein – or indirectly – by improving autophagy. Clearance of spike protein may take time, so other actions could be made to counteract immunosuppressive signalling and reinstate proper anti-pathogenic and anticancer immune function.
So, how would we do that?
Direct Clearance through Pharmaceutical Intervention
The soy fermentation–derived enzyme nattokinase can be acquired as a "soft pharmaceutical" (i.e. one not requiring doctoral oversight). Nattokinase is thrombolytic (dissolves blood clots), fibrinolytic (dissolves the protein, by which blood clots and spike proteins are aggregated), and digests both free and membrane-bound spike protein.1
It's unclear, whether nattokinase can efficiently cross the cellular membrane and unfold its spike-proteinolytic activity in the intracellular space. Computer simulations give it a high likelihood, but computer simulations of biological systems are flawed and inaccurate.
Nonetheless, nattokinase can effectively lower circulating spike protein, which would already counteract a lot of the negative effects of spike protein. Loss of free spike protein would normalise thrombotic signalling – which would in turn lower pro-inflammatory signalling –, reduce immunosuppressive signalling, lower the risk for embolisms and thrombotic hypoxia, improve blood-brain barrier function, and decrease oestrogen-disruptive signalling.
We publish new articles, whenever we've synthesised new findings to a usable degree. You can subscribe to receive articles for free.
That's already a good start to solving our problem. The application of nattokinase isn't without risk, however. As nattokinase is a thrombo- and fibrinolytic, it hampers with the natural formation of blood clots, which can lead to nose bleeds, the formation of haematoma, longer bleeding from wounds, and inner bleeding in more extreme overdoses.
In clinical settings, it's common to administer nattokinase at daily oral dosages of 2,000–4,000 FU, with the highest daily dose being 16,000 FU/d (800 mg/d).25 It's probably recommendable to stay toward the lower end of this dose range, as to minimise the above adverse effects as well as the dislodgement of thrombotic plaques, which could in turn cause embolisms.
For effective therapy, we need to tap into the positive effects of nattokinase without exposing the patient to untenable adverse effects. The logic here is, that we don't want to thin the blood, but only counteract the blood-thickening signalling of spike protein. To ensure intracellular clearance of spike protein we luckily have other tools at our disposal.
Indirect Clearance through Boosting Autophagy
Beyond using nattokinase, we can also clear spike protein via autophagy. Autophagy is a natural cellular process, where cellular or foreign components are degraded. We can increase autophagy by various means, though it's probably recommendable to use all means at once, because – as mentioned above – spike protein hampers with autophagy.
Dietary Adaptations and Fasting
The primary and simplest way, in which we can boost autophagy is by adapting our dietary lifestyle. Autophagy is induced in some tissues during ketosis. Ketosis can be achieved either by eating a diet high in fat, low in carbohydrates, and moderate in protein or by fasting for extended periods.26–27 How to do the former is extensively discussed in our Mosaic Method Guide.28
The benefit of using fasting in addition to or above normal ketogenic nutrition – which in itself is healthy and does induce autophagy – is, that fasting seems to more robustly induce autophagy in a less tissue-dependent way.29 Whilst ketosis alone does induce autophagy, it does lack the full pro-autophagic signal tone exhibited by fasting. Ketosis alone, for example, doesn't stimulate the pro-autophagic signal induced by amino acid or growth factor deprivation.30–33 This supports fasting as a more intense stimulant of autophagy across all tissues.
Fasting for extended periods is superior to shorter fasting in inducing autophagy and induction is more significant at 48 hours over 24 hours of fasting.34 One study in humans showed significant changes in the expression of two genes involved in autophagy with a time-restricted feeding pattern, where food intake was allowed for 6 continuous hours and disallowed for a following continuous 18 hours.35 It's not entirely clear how robustly such a time-restricted feeding regimen induces autophagy compared to an intermittent fasting regimen with longer fasting times. Given the fact, that autophagic induction still rises from the 24- to 48-hour mark of continuous fasting, it's probably superior to employ the longer fasting regimen. Nonetheless, one study in mice found, that given the same daily caloric intake, mice were generally healthier, when feeding was restricted to two short windows, with complete food deprivation between feedings.36 It thus seems to be wisest to combine the approaches of time-restricted eating with intermittent fasts one to four times every two weeks.
The most simple way of employing an extended fast to induce autophagy is probably to leave out a whole day of eating and skip breakfast on the following day. Given a latest food intake on day 0 at 2000 h, and a earliest food intake at 1200 h on day 2, this would give us a fasting window of 40 hours, thus solidly putting us into robust autophagic induction.
Autophagy-Stimulating Pharmaceuticals
We can also use pharmaceutical compounds to stimulate autophagy. One of the most powerful such autophagic stimulants is resveratrol, which is found in grape seeds and their extracts, but there are also other stimulants.37 Many of the pharmaceuticals we use for cancer care are used because – amongst many other benefits – they stimulate autophagy. Such other autophagic stimulants are EGCG, curcumin, and astaxanthin, as well as extracts from Ganoderma lucidum and Cordyceps sinensis.36,38–41 You can find dosage information on the former three in our Mosaic Method Guide and for the latter two in their respective posts.28,42–43
For resveratrol, dosing shouldn't exceed 450 mg/d as this has been found to be safe via preclinical validation. In controlled environments, up to 5 g/d of trans-resveratrol have been used for 28 days, so staying below 450 mg/d should ensure safety.44 Effect should be ensured at 300 mg/d, though results are varied.45 For this reason a combinatory approach using all the herein recommended therapeutic interventions is probably most helpful in clearing spike protein efficiently.
Clearance of Chronic Inflammation and Latent Infections
As mentioned above mitochondria are involved in the perpetuation of inflammation and form a kind of vicious cycle, where prolonged inflammation will damage mitochondria and damaged mitochondria will prolong inflammation, eventually chronicising it.16 In such vicious cycles, it's obviously smartest to counteract all nodes of the cycle. So, in our given vicious cycle, we'd want to reconstitute mitochondrial health and function, as well as resolve chronic inflammation.
We don't take sponsoring or display advertisements in order to keep us free from conflicts of interest. If you've found this valuable, you can help us by supporting our fight against cancer financially. Without your support we'll eventually have to terminate our efforts.
Reconstitution of Mitochondria
Energy metabolism in our cells can be divided into two principle modes: aerobic – which uses oxygen – and anaerobic – which does not.46–47 Aerobic energy metabolism can only be performed within mitochondria. If we can enforce aerobic energy metabolism, we can thus force greater reliance on mitochondria and thus create a necessity for our cells' improving mitochondrial function.
There are principally two things we can do to promote aerobic energy metabolism. We can reduce the availability of fuels, which can be metabolised anaerobically (aka "fermentation"), and we can increase the availability of fuels, which can only be metabolised aerobically (aka "respiration"). Fuels, which can be fermented, are sugar and glutamate, and fuels, which can only be respired, are fats and ketones. To improve aerobic fuel availability and minimise anaerobic fuel availability, we thus want to change dietary patterns as to exclude or minimise carbohydrates and glutamate and maximise fat. This is commonly called a ketogenic diet and is already helpful in improving autophagy. In fact, there's emerging evidence, that patients undergoing androgen deprivation therapy (ADT) – a therapeutic invention, which slakes testosterone – as treatment for prostate cancer have improved energy, less fatigue, and improve metabolic function on ketogenic diets, despite ADT being damaging to mitochondrial health and function.48–49
There is, however, more we can do for mitochondrial reconstitution.
Over their lifetimes, mitochondria take significant damage from metabolic byproducts produced during respiration. Like internal combustion engines, mitochondria can't go on forever. In internal combustion engines, we perform oil changes, gasket changes, and other repairs as the engine ages to stop it from breaking down from accruing damage. But even that will eventually not be enough, and the engine breaks down finally. Mitochondria are similar. They will constantly repair themselves and regenerate, they will fuse with one another, to improve function by balancing out the damaged components of the pre-fusion parts.50–51 But eventually, even that won't be enough, and the damaged components need to be sequestered, destroyed, and their materials recycled for the cell to continue having functional mitochondria.51
This process of recycling damaged components is known as mitophagy. Mitophagy is the specific autophagy of mitochondria – hence the name. Mitophagy turnover is controlled by various signalling pathways, but limited by general autophagic flux.30,53 Thus, increasing autophagic flux improves mitophagy. Autophagic flux can be – as discussed above – be induced via ketosis, fasting, and various pharmaceuticals.
It's not entirely clear how long mitochondrial reconstitution takes. It can thus be helpful to administer methylene blue in the short term to aid with mitochondrial respiratory capacity until they can be fully reconstituted. I've written at length about methylene blue, and about why it seems ill-advised to use it in the long run, but short-term therapeutic administration seems to be what it excels at.54
Lastly, of course, proper nourishment with those essential nutrients mitochondria require is needed to endeavour proper reconstitution of mitochondrial function. Quite a lot of nutrients are used by the mitochondria and required for their function – namely these are iron, copper, zinc magnesium, selenium, calcium, most B-vitamins, as well as vitamins C, D3, and E, as well as sulfur.55–57 There are furthermore other so-called cofactors, which mitochondria require and which are produced by cells, but that can also be supplemented, such as coenzyme Q10 and L-carnitine.
Lastly, to help our mitochondria reconstitute and remain healthy over the long term, we must resolve chronic inflammation.
Resolving Chronic Inflammation
Chronic inflammation – as mentioned above, especially of the type engendered by spike protein – is highly immunosuppressive. So clearing this specific chronic inflammation has double benefit: we improve the longevity of our mitochondrial health and we improve the activity of our immune system in its destruction of infected or otherwise compromised cells, thus being able to clear the effects of long-COVID and vaccine injuries.20,58
Principally, there are some nutrients we simply need in order for our body to even be able to clear inflammation and properly modulate the response of the immune system. Vitamins A and D3, as well as omega-3 fatty acids are essential to this.59–60 Without them, our body simply can't clear inflammation and properly modulate the immune system. A bonus to omega-3 is, that it can be used for anti-thrombotic signalling by the body.61 An added benefit of vitamins A and D3 is that they are involved in both positive and negative immune modulation, and thus allow for the immune system to adapt rapidly and precisely to changing requirements and stresses, thus acting more the scalpel than the brute's balled fist. It's obvious, why such a precise response would be beneficial in clearing latent infections, but there's more we can do.
As mentioned above, our chronic inflammation is specifically mediated by IL-6. Luckily for us, there exist fungal and plant extracts and compounds, which inhibit IL-6 signalling, which perhaps doesn't clear IL-6, but ameliorates it's effects, thus allowing our immune system to escape its suppressive signalling. Examples of such are extracts from Ganoderma lucidum, of which I have written at length priorly, and capsaicin from chilli fruits and seeds.42,62
Thus modulating our immune response should allow us to eventually clear both the chronic inflammation – also by helping our reconstituted mitochondria – and the latent infections present. And as mentioned above, the very autophagy we induce to reconstitute mitochondria also directly sequesters and destroys cell-internal spike protein.
Closing Remarks
This has been a gargantuan article.
I apologise for the technical depth, but I hope I've been able to lay out the complex intricacies simply enough as to make them understandable. Nonetheless, I want to use these closing remarks to briefly summarise if not the effect of persistent spike protein, then at least the methods we can use to clear it, so that you have a condensed reference here.
We can clear spike protein by three principle methods:
- Direct digestion through externally supplemented enzymes.
- Administration of nattokinase from fermented soy. Caution to be taken due to fibrinolytic and thrombolytic properties.
- Indirect digestion through induction of autophagy.
- Mild induction through time-restricted eating (4–8 hours eating, 16–20 fasting).
- Strong induction through intermittent fasting (8–12 hours eating, 36–40 hours fasting).
- Immune destruction of compromised cells, for which we need:
- Resolution of immunosuppressive chronic inflammation, for which we need:
- Provision of vitamins A and D3, as well as omega-3 fatty acids.
- Reconstitution of mitochondrial health, for which we need:
- Improving availability of aerobic fuels (fats, ketones).
- Lowering availability of anaerobic fuels (carbohydrates, glutamate).
- Induction of mitophagy.
- Provision of B-vitamins, as well as vitamins C, D3, and E, calcium iron, copper, selenium, zinc, manganese, magnesium, and sulfur.
- Potential supplementation of coenzyme Q10, L-carnitine, taurine, and pyrroloquinoline quinone.
- Potential short-term administration of methylene blue.
- Resolution of immunosuppressive chronic inflammation, for which we need:
It's difficult to know, whether this list is complete, as it's inferred from disparate clinical data, mechanistic evidence, and cell-biological and immunological knowledge, but I trust that this should help a vast swath of patients suffering from spike-protein pathologies incurred as a result of the virus or the vaccines meant to protect against the virus.
As such I hope this helps. Should questions arise, don't hesitate to comment or reach out.
And should you want help with implementing all of this into your life alongside the rest of the Mosaic Method to help your body fight cancer and win, don't hesitate to reach out at marchward.com/outlive.
Swift healing and lasting health to you,
Merlin L. Marquard.
References
- Halma MTJ, Plothe C, Marik P, Lawrie TA, Halma MTJ, Plothe C, et al. Strategies for the Management of Spike Protein-Related Pathology. Microorganisms [Internet]. 2023 May 17 [cited 2025 Nov 10];11(5). Available from: https://www.mdpi.com/2076-2607/11/5/1308
- Petrosino- S, Matende N. Elimination/Neutralization of COVID-19 Vaccine-Produced Spike Protein: Scoping Review. Mathews Journal of Nutrition & Dietetics. 2024 Apr 12;7(2):1–23.
- Halma MTJ, Marik PE, Saleeby YM. Exploring autophagy in treating SARS-CoV-2 spike protein-related pathology. Endocrine and Metabolic Science. 2024 Mar 31;14:100163.
- Reddan B, Cummins EP. The regulation of cell metabolism by hypoxia and hypercapnia. Journal of Biological Chemistry [Internet]. 2025 Mar 1 [cited 2025 Nov 19];301(3). Available from: https://www.jbc.org/article/S0021-9258(25)00099-7/abstract
- Ibrahim-Hashim A, Estrella V. Acidosis and cancer: from mechanism to neutralization. Cancer Metastasis Rev. 2019 June;38(1–2):149–55.
- Pillai SR, Damaghi M, Marunaka Y, Spugnini EP, Fais S, Gillies RJ. Causes, consequences, and therapy of tumors acidosis. Cancer Metastasis Rev. 2019 June;38(1–2):205–22.
- Tan Z, Chu DZV, Chan YJA, Lu YE, Rancati G. Mammalian Cells Undergo Endoreduplication in Response to Lactic Acidosis. Sci Rep. 2018 Feb 13;8(1):2890.
- Thompson SL, Bakhoum SF, Compton DA. Mechanisms of Chromosomal Instability. Current Biology. 2010 Mar 23;20(6):R285–95.
- Dai C, Sun F, Zhu C, Hu X. Tumor Environmental Factors Glucose Deprivation and Lactic Acidosis Induce Mitotic Chromosomal Instability – An Implication in Aneuploid Human Tumors. PLOS ONE. 2013 May 10;8(5):e63054.
- Perico L, Morigi M, Galbusera M, Pezzotta A, Gastoldi S, Imberti B, et al. SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation. Front Immunol [Internet]. 2022 Mar 7 [cited 2025 Nov 12];13. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2022.827146/full
- Li K, Yao L, Wang J, Song H, Zhang Y hong, Bai X, et al. SARS-CoV-2 Spike protein promotes vWF secretion and thrombosis via endothelial cytoskeleton-associated protein 4 (CKAP4). Sig Transduct Target Ther. 2022 Sept 22;7(1):332.
- Ryu JK, Yan Z, Montano M, Sozmen EG, Dixit K, Suryawanshi RK, et al. Fibrin drives thromboinflammation and neuropathology in COVID-19. Nature. 2024 Sept;633(8031):905–13.
- Srinivasan S, Guha M, Kashina A, Avadhani NG. Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochim Biophys Acta Bioenerg. 2017 Aug;1858(8):602–14.
- Hsu CC, Tseng LM, Lee HC. Role of mitochondrial dysfunction in cancer progression. Exp Biol Med (Maywood). 2016 June;241(12):1281–95.
- López-Armada MJ, Riveiro-Naveira RR, Vaamonde-García C, Valcárcel-Ares MN. Mitochondrial dysfunction and the inflammatory response. Mitochondrion. 2013 Mar 1;13(2):106–18.
- Orekhov AN, Summerhill VI, Khotina VA, Popov MA, Uzokov JK, Sukhorukov VN. Role of Mitochondria in the Chronification of Inflammation: Focus on Dysfunctional Mitophagy and Mitochondrial DNA Mutations. Gene Expression. 2023;22(4):329–44.
- Kim JA, Wei Y, Sowers JR. Role of mitochondrial dysfunction in insulin resistance. Circ Res. 2008 Feb 29;102(4):401–14.
- Cohen IR, Zanin-Zhorov A. Signaling via TLR2 and TLR4 Directly Down-Regulates T Cell Effector Functions: The Regulatory Face of Danger Signals. Front Immunol [Internet]. 2013 July 25 [cited 2025 Nov 13];4. Available from: https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2013.00211/full
- Thuya WL, Cao Y, Ho PCL, Wong ALA, Wang L, Zhou J, et al. Insights into IL-6/JAK/STAT3 signaling in the tumor microenvironment: Implications for cancer therapy. Cytokine & Growth Factor Reviews. 2025 Jan 17.
- Abdul Rahim SN, Ho GY, Coward JIG. The role of interleukin-6 in malignant mesothelioma. Transl Lung Cancer Res. 2015 Feb;4(1):55–66.
- Chen C, Chen J, Zhang Y, Zhang Q, Shi H. Senescence-associated secretory phenotype in lung cancer: remodeling the tumor microenvironment for metastasis and immune suppression. Front Oncol. 2025 May 29;15:1605085.
- Lima TM de, Martins RB, Miura CS, Souza MVO, Cassiano MHA, Rodrigues TS, et al. Tonsils are major sites of persistence of SARS-CoV-2 in children. Microbiology Spectrum. 2023 Sept 22;11(5):e01347-23.
- Seneff S, Nigh G, Kyriakopoulos AM, McCullough PA. Innate immune suppression by SARS-CoV-2 mRNA vaccinations: The role of G-quadruplexes, exosomes, and MicroRNAs. Food and Chemical Toxicology. 2022 June 1;164:113008.
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, et al. The Innate and Adaptive Immune Systems. In: Molecular Biology of the Cell. 6th edn New York, US: Garland Science, Taylor & Francis Group, LLC; 2015.; p. 1297–342.
- Hsia CH, Shen MC, Lin JS, Wen YK, Hwang KL, Cham TM, et al. Nattokinase decreases plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutrition Research. 2009 Mar 1;29(3):190–6.
- Masood W, Annamaraju P, Khan Suheb MZ, Uppaluri KR. Ketogenic Diet. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 [cited 2025 Oct 17]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK499830/
- Sanvictores T, Casale J, Huecker MR. Physiology, Fasting. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 [cited 2025 Nov 18]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK534877/
- Leonhard Marquard M, Leonhard Marquard C. The Mosaic Method [Internet]. Marchward. 2025 [cited 2025 Sept 29]. Available from: https://www.marchward.com/mosaic/
- Liśkiewicz D, Liśkiewicz A, Nowacka-Chmielewska MM, Grabowski M, Pondel N, Grabowska K, et al. Differential Response of Hippocampal and Cerebrocortical Autophagy and Ketone Body Metabolism to the Ketogenic Diet. Front Cell Neurosci [Internet]. 2021 Aug 11 [cited 2025 Nov 13];15. Available from: https://www.frontiersin.org/journals/cellular-neuroscience/articles/10.3389/fncel.2021.733607/full
- He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93.
- Alers S, Löffler AS, Wesselborg S, Stork B. Role of AMPK-mTOR-Ulk1/2 in the Regulation of Autophagy: Cross Talk, Shortcuts, and Feedbacks. Molecular and Cellular Biology. 2012 Jan 1;32(1):2–11.
- Rahmani J, Kord Varkaneh H, Clark C, Zand H, Bawadi H, Ryan PM, et al. The influence of fasting and energy restricting diets on IGF-1 levels in humans: A systematic review and meta-analysis. Ageing Research Reviews. 2019 Aug;53:100910.
- Wei Y, An Z, Zou Z, Sumpter R Jr, Su M, Zang X, et al. The stress-responsive kinases MAPKAPK2/MAPKAPK3 activate starvation-induced autophagy through Beclin 1 phosphorylation. Malhotra V, editor. eLife. 2015 Feb 18;4:e05289.
- Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-term fasting induces profound neuronal autophagy. Autophagy. 2010 Aug 16;6(6):702–10.
- Jamshed H, Beyl RA, Manna DLD, Yang ES, Ravussin E, Peterson CM, et al. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients [Internet]. 2019 May 30 [cited 2025 Nov 20];11(6). Available from: https://www.mdpi.com/2072-6643/11/6/1234
- Martinez-Lopez N, Tarabra E, Toledo M, Garcia-Macia M, Sahu S, Coletto L, et al. System-wide Benefits of Intermeal Fasting by Autophagy. Cell Metabolism. 2017 Dec 5;26(6):856-871.e5.
- Pineda-Ramírez N, Aguilera P. Resveratrol as an inductor of autophagy: is there a unique pathway of activation? Neural
- Kim SH, Kim H, Kim SH, Kim H. Astaxanthin Modulation of Signaling Pathways That Regulate Autophagy. Marine Drugs [Internet]. 2019 Sept 23 [cited 2025 Nov 20];17(10). Available from: https://www.mdpi.com/1660-3397/17/10/546
- Xiao K, Jiang J, Guan C, Dong C, Wang G, Bai L, et al. Curcumin Induces Autophagy via Activating the AMPK Signaling Pathway in Lung Adenocarcinoma Cells. Journal of Pharmacological Sciences. 2013 Jan 1;123(2):102–9.
- Peng HH, Wu CY, Hsiao YC, Martel J, Ke PY, Chiu CY, et al. Ganoderma lucidum stimulates autophagy-dependent longevity pathways in Caenorhabditis elegans and human cells. Aging. 2021 May 20;13(10):13474–95.
- Li Y, Li R, Zhu S, Zhou R, Wang L, Du J, et al. Cordycepin induces apoptosis and autophagy in human neuroblastoma SK-N-SH and BE(2)-M17 cells. Oncology Letters. 2015 June 1;9(6):2541–7.
- Leonhard Marquard M. On Ganoderma Mushrooms and Cancer Immunotherapy [Internet]. Marchward. 2025 [cited 2025 Oct 3]. Available from: https://www.marchward.com/on-ganoderma-mushrooms-and-cancer-immunotherapy/
- Leonhard Marquard M. On Cordyceps Sinensis and Its Interactions with Cancer [Internet]. Marchward. 2025 [cited 2025 Nov 20]. Available from: https://www.marchward.com/on-cordyceps-sinensis-and-its-interactions-with-cancer/
- Edwards JA, Beck M, Riegger C, Bausch J. Safety of resveratrol with examples for high purity, trans-resveratrol, resVida≫. Annals of the New York Academy of Sciences. 2011;1215(1):131–7.
- Berman AY, Motechin RA, Wiesenfeld MY, Holz MK. The therapeutic potential of resveratrol: a review of clinical trials. npj Precision Onc. 2017 Sept 25;1(1):35.
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, et al. Cell Chemistry and Bioenergetics. In: Molecular Biology of the Cell. 6th edn New York, US: Garland Science, Taylor & Francis Group, LLC; 2015.; p. 43–108.
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, et al. Energy Conversion: Mitochondria and Chloroplasts. In: Molecular Biology of the Cell. 6th edn New York, US: Garland Science, Taylor & Francis Group, LLC; 2015.; p. 753–812.
- Chi JT, Lin PH, Tolstikov V, Oyekunle T, Chen EY, Bussberg V, et al. Metabolomic effects of androgen deprivation therapy treatment for prostate cancer. Cancer Medicine. 2020;9(11):3691–702.
- Chi JT, Lin PH, Tolstikov V, Oyekunle T, Galvan GC, Ramirez-Torres A, et al. The influence of low-carbohydrate diets on the metabolic response to androgen-deprivation therapy in prostate cancer. The Prostate. 2021;81(10):618–28.
- Deretic V, Kroemer G. Autophagy in metabolism and quality control: opposing, complementary or interlinked functions? Autophagy. 2022 Feb;18(2):283–92.
- Youle RJ, van der Bliek AM. Mitochondrial Fission, Fusion, and Stress. Science. 2012;337:1062–5.
- Wang S, Long H, Hou L, Feng B, Ma Z, Wu Y, et al. The mitophagy pathway and its implications in human diseases. Sig Transduct Target Ther. 2023 Aug 16;8(1):304.
- He L, Chu Y, Yang J, He J, Hua Y, Chen Y, et al. Activation of Autophagic Flux Maintains Mitochondrial Homeostasis during Cardiac Ischemia/Reperfusion Injury. Cells [Internet]. 2022 July 4 [cited 2025 Nov 20];11(13). Available from: https://www.mdpi.com/2073-4409/11/13/2111
- Leonhard Marquard M. Marchward. 2025 [cited 2025 Nov 20]. On the Complexity of Nitric Oxide in Cancer and the Prospect of Hydrogenated Water over Methylene Blue. Available from: https://www.marchward.com/on-the-complexity-of-nitric-oxide-in-cancer-and-the-prospect-of-hydrogenated-water-over-methylene-blue/
- Wesselink E, Koekkoek WAC, Grefte S, Witkamp RF, van Zanten ARH. Feeding mitochondria: Potential role of nutritional components to improve critical illness convalescence. Clinical Nutrition. 2019 June 1;38(3):982–95.
- Ames BN. Low micronutrient intake may accelerate the degenerative diseases of aging through allocation of scarce micronutrients by triage. Proceedings of the National Academy of Sciences. 2006 Nov 21;103(47):17589–94.
- Stiban J, So M, Kaguni LS. Iron-sulfur clusters in mitochondrial metabolism: Multifaceted roles of a simple cofactor. Biochemistry (Moscow). 2016 Oct 1;81(10):1066–80.
- Alberts B, Johnson A, Lewis J, Morgan D, Raff M, Roberts K, et al. The Innate and Adaptive Immune Systems. In: Molecular Biology of the Cell. 6th edn New York, US: Garland Science, Taylor & Francis Group, LLC; 2015.; p. 1297–342.
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nature Reviews Immunology. 2008 Sept 1;8(9):685–98.
- Im DS. Functions of omega-3 fatty acids and FFA4 (GPR120) in macrophages. Eur J Pharmacol. 2016 Aug 15;785:36–43.
- Adili R, Hawley M, Holinstat M. Regulation of platelet function and thrombosis by omega-3 and omega-6 polyunsaturated fatty acids. Prostaglandins Other Lipid Mediat. 2018 Nov;139:10–8.
- Lee HK, Seo IA, Shin YK, Park JW, Suh DJ, Park HT. Capsaicin inhibits the IL-6/STAT3 pathway by depleting intracellular gp130 pools through endoplasmic reticulum stress. Biochemical and Biophysical Research Communications. 2009 May 1;382(2):445–50.