Recurrence (return of a disease after 'complete remission') or relapse (worsening of disease after 'partial remission') rates in cancer are incredibly high. To name a few examples, in triple-positive breast cancer (a very treatable cancer type) the 5-year recurrence rate was seen to be 10% in one original research paper (1). In the same paper, patients afflicted with the somewhat less treatable HER2-positive breast cancer exhibited a 5-year recurrence rate of 26.7%. These are already horribly high rates for what are considered 'treatable' cancers.
But, oh, it does get worse. In glioblastoma – which is considered the most aggressive possible brain cancer – 12-month recurrence rates were found to be as high as 83% (2).
In very few common diseases do we ever see the problem of recurrence, never mind such a deadly problem with it. Once you've gotten over a cold, you won't suddenly have a recurrence of the disease. It's thus not logical that cancer as a disease should be so different. Why should it recur even after you've gotten over it and been declared cancer-free?
The most marked difference is probably found with the widely hailed holy-grail of cancer research. Namely, every cancer researcher on the planet is still holding out hope for the cancer cure.
Why, you may ask? Well, because we've never been able to cure cancer. We've only ever been able to slow its march of death and elongate patient lives. This is, of course, already a considerable achievement, that is no doubt going to improve further as we develop cancer treatment paradigms, based on the much recent (a decade ish) development of understanding cancer as a disease of mitochondrial-metabolic origin (3–6). Though, bear in mind, that cancer does exhibit a heavy genomic-genetic component and is thus not solely a mitochondrial-metabolic disease (7).
But what even does it mean, that cancer has a 'mitochondrial-metabolic' origin?
Those are some nice words, but don't make terribly much sense in a vacuum. It's first important to understand, that our cells are internally compartmentalised, so that they have various compartments inside themselves, which fulfil various specialised functions. There are two compartments important to understanding cancer with its two axes – mitochondrial-metabolic and genomic-genetic. These are the cell nucleus and the mitochondria (compare Fig.1). The cell nucleus houses the cells primary genome and thus is here the carrier of the genomic-genetic axis of cancer, whereas the mitochondria hold the primary energy metabolism and are thus the carriers of the mitochondrial-metabolic axis.
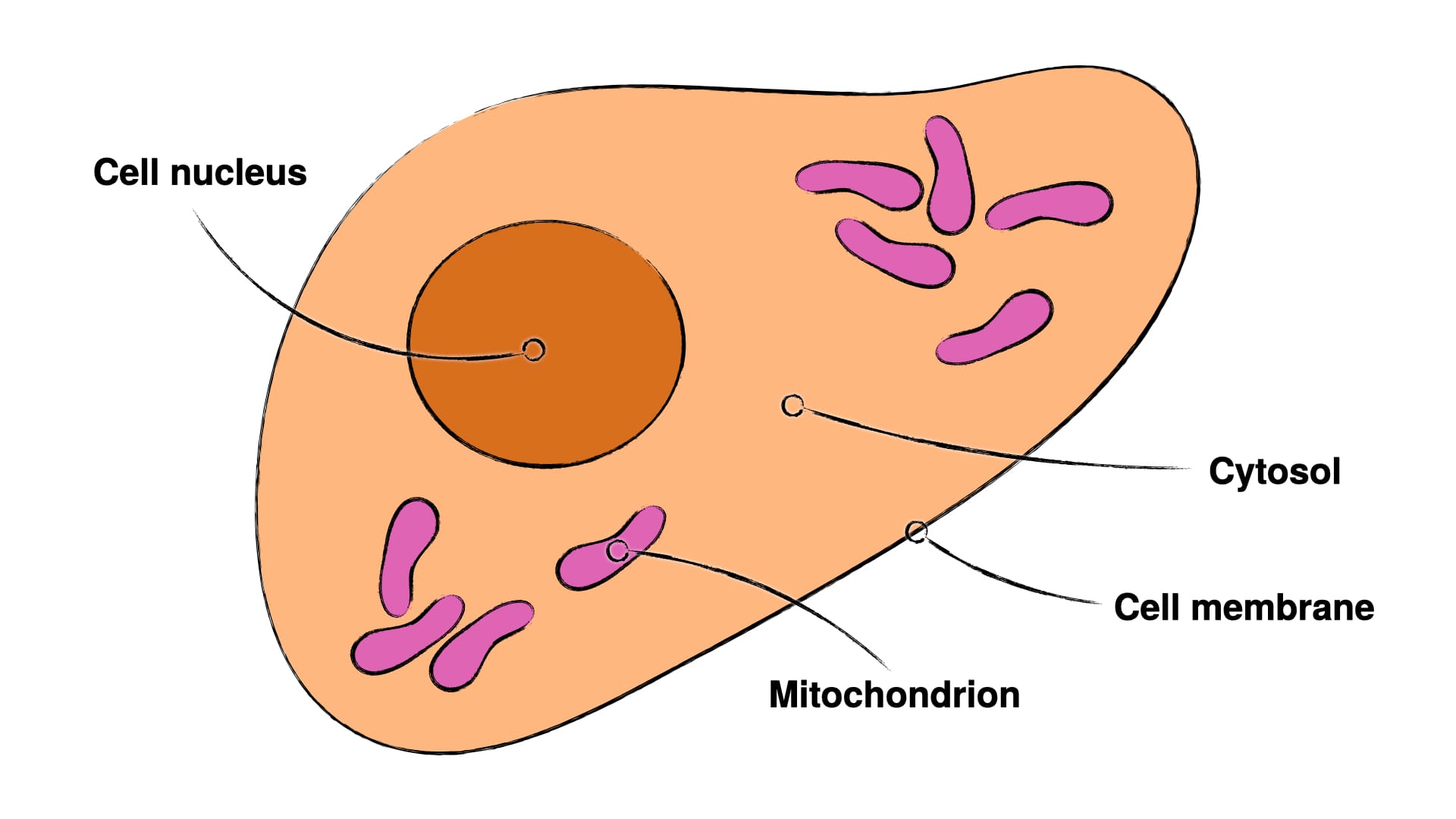
Now, what seems to happen in cancer development (bear in mind this is only my current reading of current research, which may indeed and hopefully be subject to change and improvements) is, that mitochondrial metabolism is first suppressed over an extended period of time, which can happen through carbohydrate metabolism, oxygen impoverishment, chronic inflammation, or certain toxins (like carcinogens) (8–12). Interestingly, suppressing carbohydrate metabolism has shown to reactivate mitochondrial metabolism, which indicates that we can perhaps reverse some of this mitochondrial-metabolic suppression, when dealing with cancer (13).
Mitochondrial-metabolic suppression itself doesn't give you cancer, though. Many cancers also exhibit higher-than-normal rates of genetic mutation and destabilisation of the genome (14–6). Cancer cells need these higher mutation rates to acquire the necessary genomic-genetic adaptations, which allow them to grow rapidly, spread throughout the body, and stop various failsafes built into our genomes (17). The mitochondrial-metabolic axis alone doesn't seem to effectuate that.
Nonetheless, the novel insight of the mitochondrial-metabolic axis even being a component to cancer opens up additional avenues of attack agains cancer, which may indeed enable us to better fight cancer. And we already see this bearing out in clinical studies, which have begun to find marked success in application of findings from the mitochondrial-metabolic axis of cancer.
Addition of ketogenic diet to a chemotherapeutic regimen has increased progression-free 5-year survival rate in a study of 37 patients of various cancers from 3.125% in the group, who followed a ketogenic diet for less than 12 months, to 47.62% in the group, who followed a ketogenic diet for 12 month or more (18).
This is a remarkable increase in treatment penetrance and it irks me considerably, that this hasn't been immediately adapted into every single cancer treatment regimen the world round, for the ketogenic diet isn't just good against cancer, it's simply generally healthy for humans (19–21). So, there'd be absolutely no risk in adding this to a classical medical regimen, whatsoever.
Another avenue of attack deriving from the mitochondrial-metabolic axis of cancer has been alkalisation therapy, which is borne out from the knowledge, that cancer tumours will exhibit metabolic acidosis, and that this acidosis will have far-reaching consequences – like cancer aggression through acidic retrograde signalling, immune evasion through acidic immune cell suppression, and death of tumour-surrounding cells (for this and its scientific references, see the prior article 'Helping Cancer Heal through pH Neutralisation').
Stress – both corticoid and adrenergic – has profound effects on metabolism (22–4). Perhaps unsurprisingly, the adrenergic stress system has shown effect on the tumour microenvironment, whilst patients with high corticoid stress have been associated with shorter lifespans (25–6). Keep in mind, that concerning the latter, it's not quite obvious, that this association is solely due to high corticoid stress driving worse cancer survival chances, for it could well be, that those patients with worse diagnoses also experience higher stress. Now, the study authors do remark these limitations in their pilot study, but this aspect of cancer and cancer survival is little studied and thus could offer another untapped treatment avenue.
Given the mass of treatment avenues, which have thus far not been utilised well or at all, I think you can easily see, why cancer has such a horrid recurrence rate. We've simply not been using all the tool in our tool belt. This is an issue common to all technological adaptation, where we will see a lag between innovation and major adaptation. I think the fact, that few people and corporations have financial incentive to share these new innovations, exacerbates the problem, but I'd think it disingenuous to say, that the lag is solely due to health-disinterested financial interests.
Nonetheless, the information of this creeping innovation in cancer treatment and care is out there and we at Marchward do our best to get it to you as quickly as possible. Some of the innovations are risky and shouldn't be attempted without supervision, others are perfectly safe and simply signify healthy lifestyles.
At the end of the day, I think it wise for you to use every weapon in our arsenal to fight your cancer. There's much you can do at home to increase your survival chances drastically, which have little or no risk and enormous upsides. The two principal examples of these are adapting an animal-based ketogenic diet and initiating alkalisation therapy.
If you want some pointers regarding both of these, you can find them here.
With that I wish you swift healing and lasting health.
God bless,
Merlin.
- Ma, D., Yang, Q., Yin, K., Shi, P., Chen, X., Dong, T., Shang, X., and Tian, X. (2022). Analysis of the clinicopathological characteristics and prognosis of triple-positive breast cancer and HER2-positive breast cancer-A retrospective study. Front Oncol 12, 999894. 10.3389/fonc.2022.999894.
- Kazmi, F., Soon, Y.Y., Leong, Y.H., Koh, W.Y., and Vellayappan, B. (2019). Re-irradiation for recurrent glioblastoma (GBM): a systematic review and meta-analysis. Journal of Neuro-Oncology 142, 79-90. 10.1007/s11060-018-03064-0.
- Bonnet, S., Archer, S.L., Allalunis-Turner, J., Haromy, A., Beaulieu, C., Thompson, R., Lee, C.T., Lopaschuk, G.D., Puttagunta, L., Bonnet, S., et al. (2007). A Mitochondria-K+ Channel Axis Is Suppressed in Cancer and Its Normalization Promotes Apoptosis and Inhibits Cancer Growth. Cancer Cell 11, 37-51. 10.1016/j.ccr.2006.10.020.
- Cook, K.M., Shen, H., McKelvey, K.J., Gee, H.E., and Hau, E. (2021). Targeting Glucose Metabolism of Cancer Cells with Dichloroacetate to Radiosensitize High-Grade Gliomas. Int J Mol Sci 22. 10.3390/ijms22147265.
- Di Gregorio, J., Petricca, S., Iorio, R., Toniato, E., and Flati, V. (2022). Mitochondrial and metabolic alterations in cancer cells. European Journal of Cell Biology 101, 151225. https://doi.org/10.1016/j.ejcb.2022.151225.
- Seyfried, T.N., and Chinopoulos, C. (2021). Can the Mitochondrial Metabolic Theory Explain Better the Origin and Management of Cancer than Can the Somatic Mutation Theory? Metabolites 11.
- Seth Nanda, C., Venkateswaran, S.V., Patani, N., and Yuneva, M. (2020). Defining a metabolic landscape of tumours: genome meets metabolism. British Journal of Cancer 122, 136-149. 10.1038/s41416-019-0663-7.
- Waldhart, A.N., Muhire, B., Johnson, B., Pettinga, D., Madaj, Z.B., Wolfrum, E., Dykstra, H., Wegert, V., Pospisilik, J.A., Han, X., and Wu, N. (2021). Excess dietary carbohydrate affects mitochondrial integrity as observed in brown adipose tissue. Cell Reports 36. 10.1016/j.celrep.2021.109488.
- Jiang, J., Jiang, Y., Zhang, Y.-G., Zhang, T., Li, J.-H., Huang, D.-L., Hou, J., Tian, M.-Y., Sun, L., Su, X.-M., et al. (2021). The effects of hypoxia on mitochondrial function and metabolism in gastric cancer cells. Translational Cancer Research 10, 817-826.
- Srinivasan, S., Guha, M., Kashina, A., and Avadhani, N.G. (2017). Mitochondrial dysfunction and mitochondrial dynamics-The cancer connection. Biochim Biophys Acta Bioenerg 1858, 602-614. 10.1016/j.bbabio.2017.01.004.
- Orekhov, A.N., Summerhill, V.I., Khotina, V.A., Popov, M.A., Uzokov, J.K., and Sukhorukov, V.N. (2023). Role of Mitochondria in the Chronification of Inflammation: Focus on Dysfunctional Mitophagy and Mitochondrial DNA Mutations. Gene Expression 22, 329-344. 10.14218/ge.2023.00061.
- Hsu, C.C., Tseng, L.M., and Lee, H.C. (2016). Role of mitochondrial dysfunction in cancer progression. Exp Biol Med (Maywood) 241, 1281-1295. 10.1177/1535370216641787.
- Shiratori, R., Furuichi, K., Yamaguchi, M., Miyazaki, N., Aoki, H., Chibana, H., Ito, K., and Aoki, S. (2019). Glycolytic suppression dramatically changes the intracellular metabolic profile of multiple cancer cell lines in a mitochondrial metabolism-dependent manner. Sci Rep 9, 18699. 10.1038/s41598-019-55296-3.
- Duesberg, P., Stindl, R., and Hehlmann, R. (2000). Explaining the high mutation rates of cancer cells to drug and multidrug resistance by chromosome reassortments that are catalyzed by aneuploidy. Proc Natl Acad Sci U S A 97, 14295-14300. 10.1073/pnas.97.26.14295.
- Hosea, R., Hillary, S., Naqvi, S., Wu, S., and Kasim, V. (2024). The two sides of chromosomal instability: drivers and brakes in cancer. Signal Transduction and Targeted Therapy 9, 75. 10.1038/s41392-024-01767-7.
- Thompson, S.L., Bakhoum, S.F., and Compton, D.A. (2010). Mechanisms of Chromosomal Instability. Current Biology 20, R285-R295. https://doi.org/10.1016/j.cub.2010.01.034.
- Hanahan, D. (2022). Hallmarks of Cancer: New Dimensions. Cancer Discovery 12, 31-46. 10.1158/2159-8290.Cd-21-1059.
- Egashira, R., Matsunaga, M., Miyake, A., Hotta, S., Nagai, N., Yamaguchi, C., Takeuchi, M., Moriguchi, M., Tonari, S., Nakano, M., et al. (2023). Long-Term Effects of a Ketogenic Diet for Cancer. Nutrients 15. 10.3390/nu15102334.
- Chen, S., Su, X., Feng, Y., Li, R., Liao, M., Fan, L., Liu, J., Chen, S., Zhang, S., Cai, J., et al. (2023). Ketogenic Diet and Multiple Health Outcomes: An Umbrella Review of Meta-Analysis. Nutrients 15. 10.3390/nu15194161.
- Dowis, K., and Banga, S. (2021). The Potential Health Benefits of the Ketogenic Diet: A Narrative Review. Nutrients 13. 10.3390/nu13051654.
- Luo, W., Zhang, J., Xu, D., Zhou, Y., Qu, Z., Yang, Q., and Lv, Q. (2022). Low carbohydrate ketogenic diets reduce cardiovascular risk factor levels in obese or overweight patients with T2DM: A meta-analysis of randomized controlled trials. Frontiers in Nutrition Volume 9 - 2022. 10.3389/fnut.2022.1092031.
- Thau L, G.J., Sharma S. Physiology, Cortisol. Available from: https://www.ncbi.nlm.nih.gov/books/NBK538239/.
- Herman, J.P., McKlveen, J.M., Ghosal, S., Kopp, B., Wulsin, A., Makinson, R., Scheimann, J., and Myers, B. (2016). Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol 6, 603-621. 10.1002/cphy.c150015.
- Sobel, B.E., Jéquier, E., Sjoerdsma, A., and Lovenberg, W.T. (1966). Effect of Catecholamines and Adrenergic Blocking Agents on Oxidative Phosphorylation in Rat Heart Mitochondria. Circulation Research 19, 1050–1061.
- Eng, J.W., Kokolus, K.M., Reed, C.B., Hylander, B.L., Ma, W.W., and Repasky, E.A. (2014). A nervous tumor microenvironment: the impact of adrenergic stress on cancer cells, immunosuppression, and immunotherapeutic response. Cancer Immunol Immunother 63, 1115-1128. 10.1007/s00262-014-1617-9.
- Cash, E., Beck, I., Harbison, B., Albert, C., and Sephton, S.E. (2024). Evening cortisol levels are prognostic for progression-free survival in a prospective pilot study of head and neck cancer patients. Frontiers in Oncology Volume 14 - 2024. 10.3389/fonc.2024.1436996.